Affected by the external environment, some environmental deposits are often produced on the surface of the hot end components of heavy-duty gas turbines (such as turbine blades), mainly from molten salts in the marine environment, vanadates and sulfates, the combustion products of low-grade fuels, and sand, fly ash, runway debris in the air, etc. These deposits have a great impact on the life of the coating and will penetrate into the coating at high temperatures, thereby causing molten salt corrosion. Therefore, molten salt corrosion is difficult to avoid in the actual application of thermal barrier coatings for heavy-duty gas turbines.

In gas turbines, thermal corrosion is an important failure mode. It is mainly caused by the oxidation of impurities S and some alkali metals in the fuel under high-temperature combustion, and reacting with NaCl, KCl and other substances in the environment to form some molten salt mixtures that adhere to the blade surface, thereby corroding the blade and affecting the blade life. Therefore, it is extremely important to improve the thermal corrosion resistance of the coating. CHEN et al. [65] studied the thermal corrosion resistance of Sc2O3-doped YSZ in Na2SO4/V2O5 (mass fraction 50%:50%) and found that ScYSZ with a higher Sc2O3 content has better thermal corrosion resistance. Yang Yingfei et al. [66] prepared four typical bonding layers, including NiAl coating, NiCoCrAlY coating, Pt-modified NiAl coating and Pt+Hf co-modified NiAl coating, and then coated them with Na2SO4/NaCl (mass fraction 75%∶
25%) and conducted hot corrosion tests at 900℃. The results showed that the Pt-modified NiAl coating had the best hot corrosion resistance. CAI et al. [67] used plasma pulse electron beam (HCPEB) to irradiate the CoCrAlY bonding layer and coated it with Na2SO4/K2SO4 (mass fraction 75%∶25%) and conducted hot corrosion tests at 900℃. The results showed that irradiation promoted the formation of Al2O3 film in the coating, thereby improving the hot corrosion resistance of the CoCrAlY coating.
CMAS corrosion[68-70] is caused by impurity particles such as sand, dust, and volcanic ash ingested from the air during the use of gas turbines. These impurity particles are mainly composed of CaO, MgO, Al2O3, SiO2, etc., so they are called CMAS. In addition to CaO, MgO, Al2O3, SiO2, etc., CMAS usually contains a small amount of other components, such as Fe2O3 and MgO, so the composition of CMAS is relatively complex. In addition, studies have found that the composition of CMAS will change due to external factors such as location, environment, and climate, causing its melting point to change accordingly, usually within a range (1150~1250℃)[70-72].
CMAS Corrosion Mechanism
CMAS is very harmful to thermal barrier coatings and seriously affects the life of the coating, as shown in Figure 7. At low temperatures, the deposited CMAS adheres to the thermal barrier coating of the turbine blades and continuously impacts the coating, causing the coating to wear or even fail [72-73]; at high temperatures, CMAS melts and has two main effects on the YSZ thermal barrier coating, namely, thermochemical and thermomechanical effects.
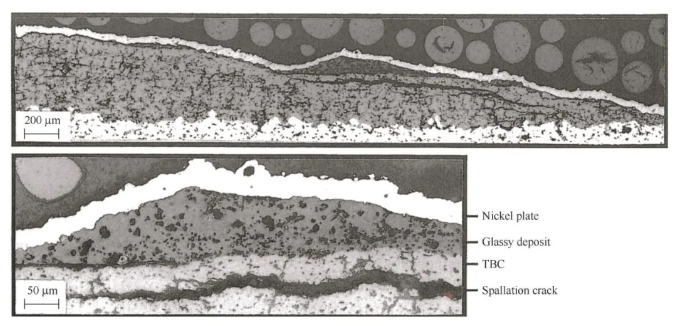

Thermomechanical aspects[74-75]: After CMAS melts, it penetrates into the ceramic layer under capillary action and solidifies after cooling, filling the pores and cracks of the coating, making the coating denser. The densification of the coating causes the following four damages: first, it reduces the stress tolerance of the coating, increases the Young’s modulus of the coating[76], and accelerates the spalling failure of the coating; second, it reduces the porosity of the coating and increases the thermal conductivity of the coating[77], resulting in a decrease in thermal insulation performance; third, there is a large difference in the thermal expansion coefficient between the CMAS-infiltrated area and the non-infiltrated area, which promotes thermal stress in the coating during the cooling process and accelerates the spalling of the coating; fourth, it increases the hardness of the coating[78] and reduces the insulation performance of the coating.
Thermochemical aspects[69, 79]: CMAS penetrates into the ceramic layer at high temperature and corrodes the coating mainly through dissolution-reprecipitation, that is, CMAS reacts with the ceramic layer to dissolve YSZ in CMAS, and then reprecipitates to form new crystals. On the one hand, the Y-O and Zr-O bonds in the YSZ grains are destroyed during the dissolution process, resulting in an increase in the diffusion rate of Y and Zr. Since the diffusion coefficient of Y ions is larger than that of Zr[80-81], more Y is separated from the YSZ grains, resulting in a lower Y content in some grains. On the other hand, since the solubility of Y2O3 in molten CMAS is higher than that of ZrO2[82], that is, CMAS and YSZ react to consume more Y2O3, the reprecipitated crystals have a high ZrO2 content and very little Y2O3 content. Therefore, these YSZ crystals with less Y content have reduced phase stability due to the lack of stabilizer, and transform from t phase to m phase, causing a 3% to 5% volume change in the coating, which in turn changes the stress distribution in the coating and causes the coating to fail. In addition, the degree of reaction in different regions is different, and the generated products are different, resulting in different thermal physical properties such as thermal expansion coefficient and elastic modulus, which aggravates thermomechanical failure and causes the coating to fail prematurely.
