The operating environment for hot-section components of shipboard aircraft engines and gas turbines is extremely harsh. In addition to enduring high temperatures, pressures, and rotational speeds, these components are also subject to the corrosive influences of a maritime environment characterized by high salinity and humidity.
Turbine rotor components represent the most critically stressed elements within these engines and turbines. These parts must bear significant alternating loads at temperatures ranging from 600 to 1300°C while also being subjected to thermal corrosion from hot exhaust gases and the marine atmosphere, which significantly increases their damage rate. As a result, turbine rotor components are among the highest failure-rate operational parts.
As shown in Figure 1, turbine blades undergo thermal corrosion-fatigue failure in the coupled environment of hot exhaust gases and marine air, under conditions of high temperature and high-speed loading. Unlike fatigue failure induced by singular mechanical loads, the mechanism of thermal corrosion-fatigue failure in the hot sections of aviation engines and gas turbines is far more intricate, thus presenting greater challenges for predicting thermal corrosion-fatigue life. To date, there has been some initial understanding both domestically and internationally regarding the mechanisms of thermal corrosion-fatigue failure in these hot sections. Research has been carried out on the mechanisms of thermal corrosion for hot-section materials, thermal corrosion-fatigue failure mechanisms, and thermal corrosion-fatigue life prediction, achieving some preliminary results.

Thermal corrosion mechanism of hot end component materials
Nickel based superalloys have become the main materials for hot end components such as aircraft engines and gas turbine blades, turbine disks, etc. due to their excellent fatigue performance, creep performance, and durability at high temperatures [7,8,9] [10,11,12]. At the same time, in order to reduce the surface temperature of turbine blades and improve their oxidation/corrosion resistance, metal coatings or thermal barrier coatings (TBC) are usually sprayed on the surface of turbine blades [13,14]. During the service of the engine, impurities S in the fuel will produce sulfides such as SO2 and SO3 during combustion. After reacting with NaCl in the marine atmospheric environment, a layer of Na2SO4 molten salt film will be deposited on the surface of the alloy. The sulfate and other deposits formed will cause thermal corrosion of the coating and high-temperature alloy, ultimately leading to premature failure of the engine’s hot end components
Two machine knowledge and key technologies!
- Hot corrosion of high-temperature alloys refers to the behavior in which sulfate and other deposits deposited on the surface of the alloy damage surface oxides and accelerate the corrosion of the alloy [17]. For high-temperature alloys, the damage caused by hot corrosion is much more severe than that caused by simple high-temperature oxidation. Its severity is influenced by multiple factors, including temperature, type and content of deposited salts, environmental conditions, gas composition, and alloy composition. According to the ambient temperature, hot corrosion can be divided into low-temperature hot corrosion and high-temperature hot corrosion [18]. Low temperature hot corrosion (560-815 ℃) generally occurs below the melting point of the corrosive salt, with the limitation of requiring a higher partial pressure of gas-phase SO3 to react with alloy elements in the coating or substrate to form new sulfates. These sulfates react with Na2SO4 to form low melting point (melting point of 540 ℃) eutectic compounds, causing localized pitting corrosion of the alloy.
- High temperature hot corrosion (815-980 ℃) usually occurs at temperatures above the melting point of the corrosive salt. Molten alkali metal salts deposit on the surface of the substrate, gradually damaging the oxide layer and consuming Cr in the substrate metal. As Cr element is depleted, the oxidation rate accelerates, and fine pores begin to form inside the coating or substrate, providing channels for the infiltration of corrosive media.
- At this point, the deposited salt in a molten state causes uniform corrosion of the alloy, and the interface between the high-temperature hot corrosion oxide layer and the alloy substrate is relatively flat, with obvious sulfide formation below the interface. Rapp [19] and Singh et al. [20] summarized the hot corrosion mechanism of metal materials, especially iron-based and nickel based high-temperature alloys, in the early 21st century. Therefore, this article mainly reviews the hot corrosion mechanism of high-temperature alloys and coatings for turbine disks and turbine blades in the past 20 years.
Coating Hot Corrosion Mechanism
According to the specific service conditions and turbine front temperature of aircraft engines and gas turbines, commonly used coatings include aluminum infiltration coatings [21] (such as aluminum infiltration on blade surfaces and inner cavities, aluminum infiltration silicon coatings), MCrAlY coatings [22], and thermal barrier coatings [23]. The typical thermal barrier coating for high-temperature alloy blades is usually composed of a metal bonding layer and a heat-insulating ceramic layer, providing insulation and anti-oxidation layers between the high-temperature alloy blade substrate and the high-temperature gas, thereby ensuring the safe operation of the blade at higher turbine front temperatures. The metal bonding layer can improve the bonding strength between the base alloy and the surface ceramic layer. During high-temperature service, it can also form a dense layer of thermally grown oxide (TGO), which plays a role in resisting high-temperature oxidation. The commonly used TBC materials currently include Y2O3 stabilized ZrO2 (YSZ, Y2O3 mass fraction is generally 7% to 8%), mullite, Al2O3, YSZ+CeO2, La2Zr2O7, silicates, among which YSZ is the most widely used TBC material [21,24,25].
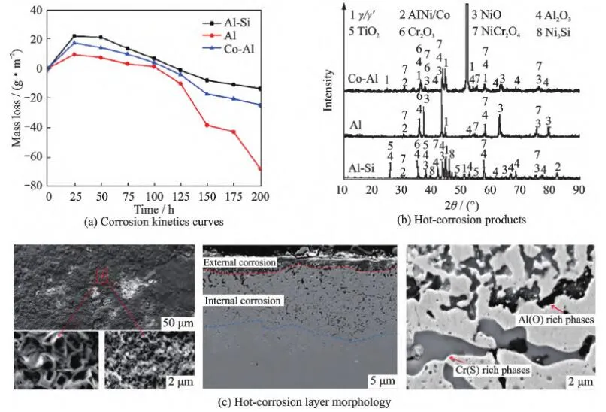
Due to the high temperature (>800 ℃) that the surface coating of turbine blades can withstand during service, the coating usually undergoes high-temperature thermal corrosion under coupled gas and marine environments. The thermal corrosion products also vary depending on the type, composition, and microstructure of the coating. For metal coatings, their thermal corrosion resistance is closely related to the formation of a continuous and dense Al2O3 protective oxide layer on the coating surface. Li Yuchun [26] studied the hot corrosion behavior of K488 alloy and its surface aluminum infiltrated and cobalt infiltrated aluminum coatings using the buried salt method. It was found that a mixture of Al2O3 and CoCr2O4 was formed on the surface of the aluminum infiltrated coating in 25% NaCl+75% Na2SO4 molten salt at 900 ℃. The presence of the mixed oxide improved the corrosion resistance, while the cobalt infiltrated aluminum coating exhibited excellent thermal corrosion resistance due to the formation of a dense Al2O3 protective oxide film on the surface. Li Yanming et al. [27] prepared three types of coatings, Al Si, Al, and Co Al, on the commonly used material DSM11 nickel based high-temperature alloy for gas turbine blades. They studied the performance of the three coatings by coating salt (mass fraction of 5% NaCl+95% Na2SO4) on the surface of the coatings.
The hot corrosion performance at 900 ℃ is shown in Figure 2. The research results indicate that the kinetic curves of the three coatings are basically similar, all showing parabolic shapes. With the extension of corrosion time, the mass first increases and then decreases. The reason for the above quality changes is that in the early stage of the corrosion experiment (0-25 hours), the corrosion products are tightly bound to the coating and have less detachment; In the middle and later stages of corrosion, surface corrosion products fall off, and the exposed internal coating or substrate further undergoes thermal corrosion, resulting in a decrease in quality. After 200 hours of hot corrosion at 900 ℃, a continuous and dense oxide layer mainly composed of Al2O3 was formed on the surface corrosion zone of both Al Si coating and Co Al coating, which inhibited the progress of hot corrosion and had good thermal corrosion resistance; Unlike Al Si coating and Co Al coating, Al coating forms a mixed oxide layer on the surface corrosion zone, and the hot corrosion reaction continues, resulting in relatively poor thermal corrosion resistance. In addition, Liu Delin et al. [28] also confirmed the anti-oxidation and corrosion effects of NiCoCrAlYTa coating on DZ22B alloy and DZ22B alloy with NiCoCrAlYTa coating at 950 ℃ through hot corrosion tests. This is based on the formation of dense Al2O3 or Cr2O3 oxide films on the surface of the coating, which act as oxygen barrier layers (shielding layers) to prevent further oxidation or corrosion of the substrate.

Similar to metal coatings, the thermal corrosion resistance of thermal barrier coatings largely depends on the continuous Al2O3 or Cr2O3 oxide thin film formed on the surface. As Li Faguo et al. [29] pointed out in their recent research progress on the resistance of high-temperature coatings for aircraft engines to marine atmospheric corrosion, the thermal corrosion resistance effect of YSZ mainly comes from dense Al2O3, among which Cr, Ta, and Y can stabilize the generation of Al2O3 and improve the thermal corrosion resistance of the coating. However, foreign Na, V, and S can cause the formation of YVO4 in the Y element, leading to the degradation of the YSZ coating. When Na2SO4 molten salt exists alone, it does not undergo a chemical reaction with YSZ. The corrosion mechanism is mainly that Na2SO4 molten salt adheres to the pores and cracks of YSZ, and the thermal stress caused by the difference in expansion coefficient during repeated cold and hot cycles leads to the failure of YSZ coating [20,21,2,23,24,25,26,27,28,30], causing Na2SO4 molten salt to penetrate into the bonding layer through TGO-YSZ, react with the bonding layer, and form a loose sulfide layer under the TGO layer. There are cracks between the TGO layer and the sulfide layer [29], ultimately leading to coating peeling off. When V2O5 molten salt is present, it reacts with stabilizer Y2O3 to generate YVO4, as shown in equation (1). The growth of YVO4 dendrites generates crack propagation stress [28,30,31]; In addition, YSZ undergoes a transition from tetragonal to monoclinic crystal structure, accompanied by a volume expansion of 3% to 5%. The coupling effect of phase transition stress and growth stress leads to coating cracking or even peeling.

- When Na2SO4 coexists with V2O5 to form NaVO3, as shown in equation (2), according to the Lewis acid base theory, compared to the acidity of V2O5, NaVO3 also has alkalinity. Therefore, compared to the corrosion process of pure V2O5, when Na2SO4 and V2O5 coexist, i.e. NaVO3 molten salt, the corrosion rate is faster, the YVO4 dendrite size is larger, and the YSZ thermal barrier coating fails faster.
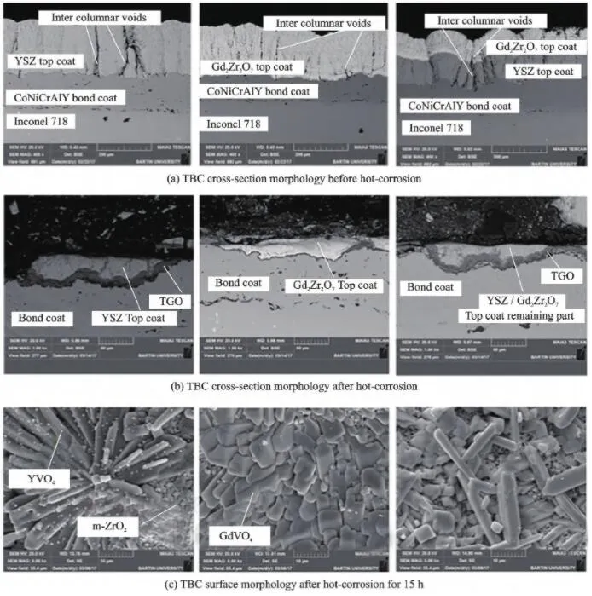
- In order to further improve the performance of thermal barrier coatings, scholars at home and abroad have studied and designed double-layer structure thermal barrier coatings, and evaluated their thermal corrosion resistance. The double-layer structure thermal barrier coating is mainly applied to new ceramic materials with poor mechanical properties. A YSZ coating is prepared between the bonding layer and the new material ceramic layer to reduce the difference in thermal expansion coefficient between layers and alleviate internal stress of the coating. Ozgurluk et al. [31] investigated the hot corrosion behavior of YSZ, Gd2Zr2O7, and YSZ/Gd2Zr2O7 thermal barrier coatings in molten sulfate and vanadate (55% V2O5+45% Na2SO4) at 1000 ℃, as shown in Figure 3. Research has found that high levels of V2O5 accelerate coating damage, and YSZ/Gd2Zr2O7 double-layer thermal barrier coatings exhibit better thermal corrosion resistance. The corrosion products in YSZ coating are monoclinic ZrO2 and YVO4, while in Gd2Zr2O7 and YSZ/Gd2Zr2O7 thermal barrier coatings, the corrosion products are monoclinic ZrO2 and GdVO4. The transformation of ZrO2 from tetragonal to monoclinic crystal structure causes damage to the coating, and the molten salt damages the columnar morphology of the coating, reducing its thermal insulation effect.
Hot corrosion mechanism of high-temperature alloys for turbine blades
When the coating of turbine blades peels off due to thermal corrosion and mechanical loads, the exposed high-temperature alloy substrate will further undergo thermal corrosion in gas and marine environments [32]. At present, aircraft engine turbine blades mainly include DD6, CMSX-4, CMSX-10, PWA1484 single crystal high-temperature alloy blades, while gas turbine turbine blades include single crystal, directionally solidified, and cast high-temperature alloy blades, such as IN-738, DZ125, K444, GTD-111, MD2, etc. [33].
In high-temperature corrosive environments, single crystal high-temperature alloys exhibit stronger resistance to thermal corrosion and lower average corrosion rates. The average hot corrosion rate of DD15 [34] single crystal high-temperature alloy in a high-temperature (900 ℃) corrosive environment mixed with fuel and seawater mist is 0.071 g/(m2 · h), and the corrosion layer does not peel off. Research on single crystal materials such as DD10 and DSM11 has shown [35] that after undergoing high-temperature hot corrosion, continuous layered protective oxide films of Cr2O3, TiO2, and Al2O3 will appear inside the corrosion products of single crystal high-temperature alloys. The corrosion products on the surface of these materials will provide good protection for the substrate, thereby hindering further hot corrosion and improving the thermal corrosion resistance of single crystal materials. In low-temperature hot corrosion environments, Luthra [36] found that Na2SO4 coated on the surface of cobalt based high-temperature alloys is solid, and sufficient SO3 partial pressure in the atmosphere can sulfide NiO to form NiSO4. This compound will react with excess Na2SO4 to form liquid eutectic salts. The molten mixed sulfate salt dissolves the protective oxide layer and rapidly transports the reactants through the liquid in the corrosion pit, increasing the corrosion rate. In nickel based alloys, the sulfation of NiO is relatively less. Lortrakul et al. [37] found that after coating Na2SO4 on the surface of CMSX-4 and heating it to 700 ℃ in an O2-SO2-SO3 atmosphere, XRD analysis showed that NiSO4 had disappeared after 5 hours of corrosion, indicating that low melting eutectic salts could no longer form. However, after 50 hours, obvious sulfide enrichment zones were observed near the external/internal corrosion layer interface and the internal corrosion layer/substrate interface, indicating that the essence of low-temperature hot corrosion is still the sulfide oxidation in the local melting zone, similar to the mechanism of high-temperature hot corrosion.
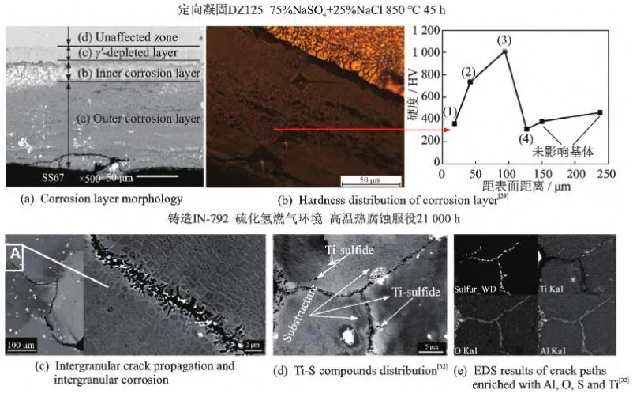
Compared to single crystal high-temperature alloy blades, directional solidification high-temperature alloys have a small amount of grain boundaries, which can cause a decrease in the alloy’s resistance to thermal corrosion. Kumawat et al. [38] evaluated the hot corrosion behavior of directionally solidified CM247LC alloy at 950 ℃ with different salt deposition amounts. Compared with pure oxidation in air, it was found that the alloy underwent accelerated corrosion degradation in a molten salt environment. Within the first 0.5 hours of exposure, the alloy reacted with oxygen elements in the molten salt to form oxides of Al and Cr, exhibiting a lower corrosion rate. Afterwards, with the increase of oxygen flux, the corrosion acceleration stage is entered, and the corrosion mechanism shifts from alkaline corrosion mechanism to acidic corrosion mechanism. Due to the infiltration of sulfur, the hot corrosion behavior can continue continuously. Yang et al. [39] conducted a study on high-temperature hot corrosion of directionally solidified DZ125 alloy at 850 ℃. The corrosion layer of the alloy is divided into two layers: the outer layer is a porous layer of oxide NiO/Cr2O3/Al2O3, while the inner layer is a uniform oxide mainly composed of Cr2O3, as shown in Figure 4. High temperature hot corrosion not only exacerbates the formation of surface oxides in directionally solidified alloys, but also leads to the formation of low strength γ ‘poor zones on the subsurface, as shown in Figure 4 (b). This is also one of the main reasons for the degradation of mechanical properties of alloys caused by high-temperature hot corrosion.
Compared to single crystal and directionally solidified high-temperature alloys, cast high-temperature alloys have more grain boundaries, and elements forming oxides and sulfides can rapidly diffuse along the grain boundaries, accelerating hot corrosion and oxidation [40]. When casting high-temperature alloys, sulfur elements diffuse along grain boundaries during long-term corrosion processes, causing intergranular brittleness that has a significant impact on their service performance. As shown in Figure 4 (d, e), IN-792 cast high-temperature alloy blades [32] exhibited mechanical and chemical damage after approximately 21000 hours of service. Among them, high-temperature hot corrosion leads to the presence of Ti sulfides and free forms of sulfur elements at grain boundaries, resulting in intergranular embrittlement and reduced resistance to crack propagation. The investigation found that almost all cracks on the IN-792 turbine blades propagate in a transgranular manner. Under the high-temperature hot corrosion of casting high-temperature alloy K35 [41] containing NaCl mixed salt, the originally dense Cr2O3 oxide layer becomes loose, providing a fast channel for the diffusion of oxygen and sulfur.